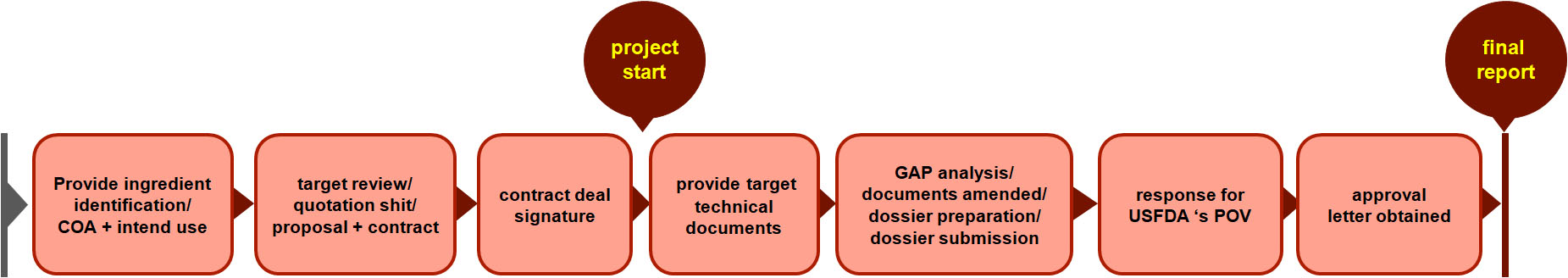
Assistance with USFDA regulations
Novel food ingredient approval with USFDA
Any dietary ingredient that was not marketed in the United States in a dietary supplement before October 15, 1994. A Premarket Notification for a New Dietary Ingredient (NDI) filed with USFDA for approval is needed.
Under sections 201(s) and 409 of the Federal Food, Drug, and Cosmetic Act (the Act), any substance that is intentionally added to food is a food additive, that is subject to premarket review and approval by FDA, unless the substance is generally recognized, among qualified experts, as having been adequately shown to be safe under the conditions of its intended use, or unless the use of the substance is otherwise excepted from the definition of a food additive.
The use of a food substance may be GRAS either through scientific procedures or, for a substance used in food before 1958, through experience based on common use in food Under 21 CFR 170.30(b), general recognition of safety through scientific procedures requires the same quantity and quality of scientific evidence as is required to obtain approval of the substance as a food additive.
Ingredient Regulatory Compliance Services
- New Additives
- NDI
- GRAS
www.e-sinew.com
contact us via whatsapp
