Dawn is coming for GMO derivatives in Taiwan
GMO derived Astaxanthin from a local company and GMO derived HMO from an international company have been successively approved by TFDA as non-traditional food ingredients last year. The former one had been applied for more than 5 years while the later had been applied for over 3 years. Both had gone through tough application process with TFDA on listing as non-traditional food ingredient. We had been involved in the process for the later one.
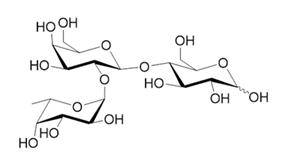
Both touched down almost the same time indicates that the attitude of TFDA officers is turning to open the door (even is not widely) to GMO derivatives. This is good news for those ingredients (compounds) with GMO metabolic process (non-traditional process) to be employed for foods and beverages since this application process got model to follow now.
SCG had plenty experience to communicate with TFDA officers and to help international clients to prepare the proper dossier for application steps. Safety issue would be the critical concern and so safety assessment would be the key parts for the dossier. The approvals in other countries would also help as the important reference for TFDA review committee and officers.
Welcome to contact us via whatsapp or email to Dr. Newton Yau via newton.yau@e-sinew.com