Friendly regulatory environment in Taiwan for medical devices
In our previous article [USING TAIWAN AS THE TEST MODEL BEFORE ENTERING INTO MAINLAND CHINA MARKET ], we had mentioned that to choose Taiwan as the testing zone for foreign companies before entering the growing Chinese market is recommended. This is especially perfect for medical devices industry as the regulatory environment for Taiwan FDA is quite friendly. Taiwan FDA would accept certain certifications issued by certain third parties on her list in order to encourage the innovative inventions on medical devices.
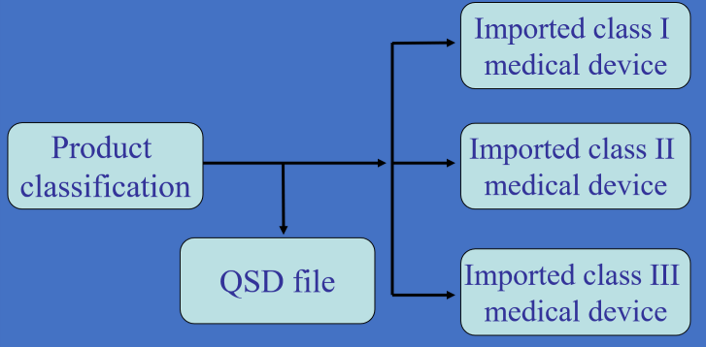
International companies can take this advantage as the first step. The modernized Pharmaceutical Affairs Law for medical devices will be easier for the player to get on the market.
Medical Device Regulatory Affairs:please click Assistance with Taiwan FDA Regulations
We also can help the Assistance with SAMR/CFDA Regulations
